Welcome to the official website of Yercon Diagnostic Co.,Ltd!
Contact Us
Address: No.2 Building, Ledong Industrial Estate 3248 Century Ave.Tech.& Economic Development Zone Changchun
Water Test strips For Aquaculture /Aquarium (6-in-1)
Category:
Detailed
Water Test strips For Aquaculture /Aquarium (6-in-1)
Intended Use:
This product can be used for the detection of nitrate (nitrate+nitrite), nitrite, residual chlorine, total hardness, carbonate, and pH in aquariums, aquaculture water, or other water quality (drinking water and its source, well water, surface water, swimming pool water, hot spring water, mineral water, sewage, etc.).
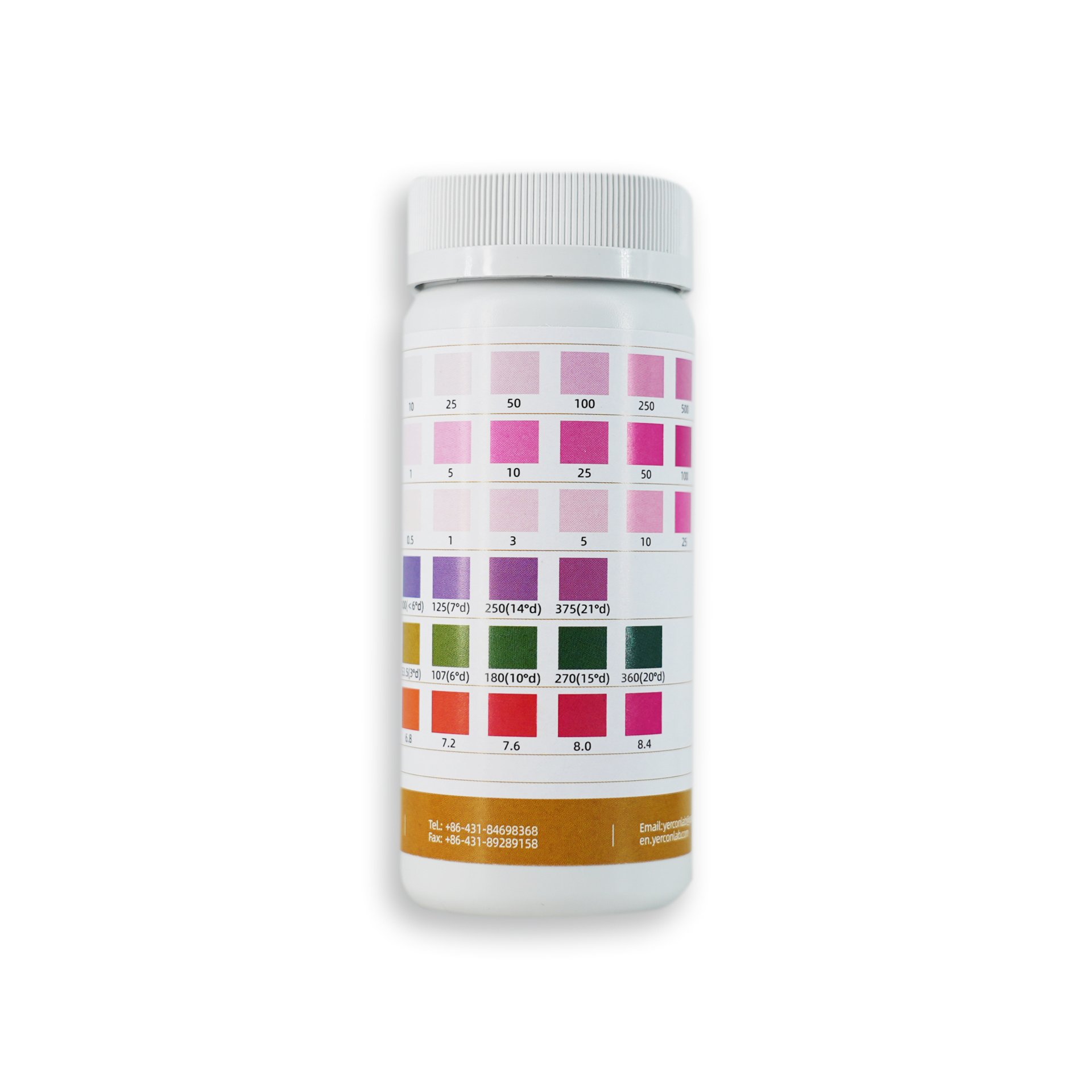
Product Testing Scope
|
Item |
Detection Concentration Range |
unit |
|||||||
|
Nitrate+Nitrite(NO3-+NO2- ) |
0 |
10 |
25 |
50 |
100 |
250 |
500 |
|
mg/L |
|
Nitrite(NO2-) |
0 |
1 |
5 |
10 |
25 |
50 |
100 |
|
|
|
Residual Chlorine(Cl2) |
0 |
0.5 |
1 |
3 |
5 |
10 |
25 |
50 |
|
|
Total Hardness(GH) |
0 |
<6° |
7° |
14° |
21° |
|
|
|
d |
|
0 |
100 |
125 |
250 |
375 |
|
|
|
mg/L |
|
|
Carbonate(KH) |
0 |
3° |
6° |
10° |
15° |
20° |
|
|
d |
|
0 |
53.5 |
107 |
180 |
270 |
360 |
|
|
mg/L |
|
|
pH |
6.4 |
6.8 |
7.2 |
7.6 |
8.0 |
8.4 |
|
|
|

Reagent Area Information
Nitrite (NO2-) and Nitrate (NO3-)
The decomposition of organic nitrogen compounds from feces, dead plants, food residues or similar takes place in several stages:
1. Ammonia and ammonium are produced from organic waste. Ammonium is taken up by plants as nitrogen fertilizer. Ammonia is highly toxic. The conversion of ammonium or ammonia depends on the pH value. Ammonium is produced at low pH levels. Ammonia is produced at high pH levels. Therefore, there is no risk of ammonia poisoning in an aquarium or garden pond with a low pH value.
2. Nitrite, a substance toxic to fish, is produced from ammonium/ammonia.
3. Nitrite is converted into nitrate. Nitrate is toxic only in high concentrations and is taken up by aquatic plants as a nutrient.
The individual decomposition stages are completed by microorganisms. An increased level of nitrite or nitrate indicates that the biological balance is disturbed. In this case, you should perform a partial water change and investigate the cause.
You can find out the nitrate content by comparing the nitrate measurement of the test strip with the corresponding color. The transition color indicates an intermediate value. The nitrate content should not exceed 25 mg/L. Aquatic plants absorb nitrates as nutrients. Therefore, adequate plant growth prevents excessive nitrate levels.
Residual Chlorine
Chlorine is added to tap water regularly for disinfection purposes. Ornamental fish and other aquarium pets cannot survive even very small amounts of chlorine, so it needs to be removed from the water added to the aquarium. Chlorine can be removed from the water by enhanced aeration or activated carbon filtration.
Total Hardness(GH)
When determining general hardness, we measure the calcium and magnesium salts dissolved in the water. Most fish prefer water with medium hardness. However, East African ornamental fish live in water with high hardness.
Carbonate (KH)
Carbonate hardness indicates the pH buffering capacity of water. It stabilizes the pH value. Alkalinity and pH affect each other in terms of their lethal effects on fish. At the same alkalinity, the higher the pH value, the stronger the toxic effect on fish.
pH
The pH value indicates the acidity level of the water. A pH of 7 is neutral. A pH below 7 is
acidic, and a pH above 7 is alkaline. Avoid sudden large changes in pH.
It is impossible to give general recommendations for ideal pH values. For example, ornamental fish from South America live in water with an acidic pH below 7, and perch fish from inland lakes in East Africa live in alkaline water with a pH above 7.
Water with a carbonate content below 3 dkH will result in inaccurate pH determinations. This will be very rare in an ordinary aquarium. In this case, adjust the carbonate hardness of the water to be tested to above 3 dkH, and then determine the pH.
Key words:
Previous Page:
Online Inquiry
Note: Please leave your phone number or E-mail and our professionals will contact you as soon as possible!
Recommend products
Contact Us
Address: No.2 Building, Ledong Industrial Estate 3248 Century Ave.Tech.& Economic Development Zone Changchun
OUR PRODUCTS
Online consultation